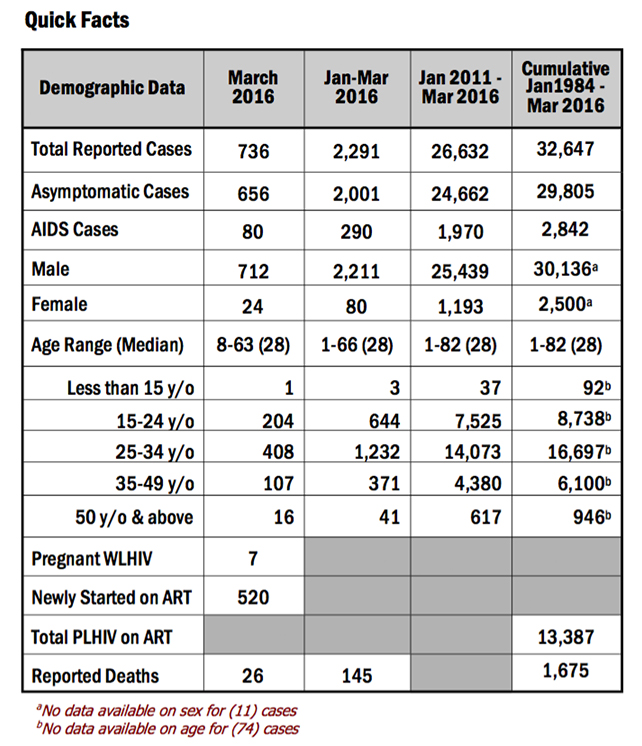
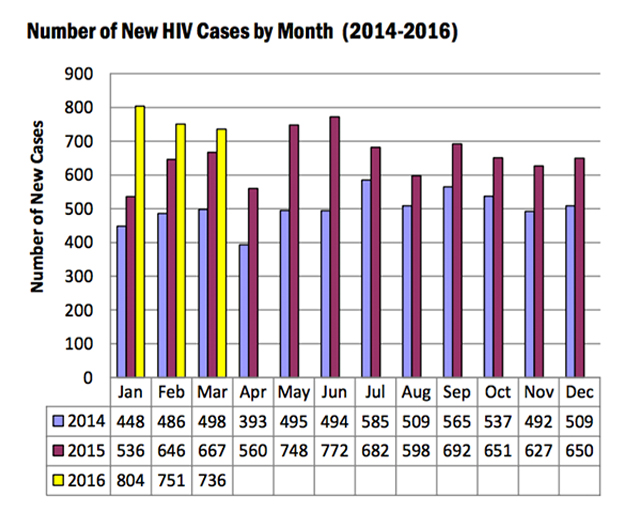
Seven hundred and thirty-sex (736) new HIV Ab seropositive individuals were reported to the HIV/AIDS & ART Registry of the Philippines this March, a number that – while lower than the February figure of 751 – was still 10% higher compared to the same period last year (667). Of the new cases, most (97%) were male, with the median age of 28 years old (age range: eight years-63 years). More than half belong to the 25-34 year age group while 27% were youth aged 15-24 years.
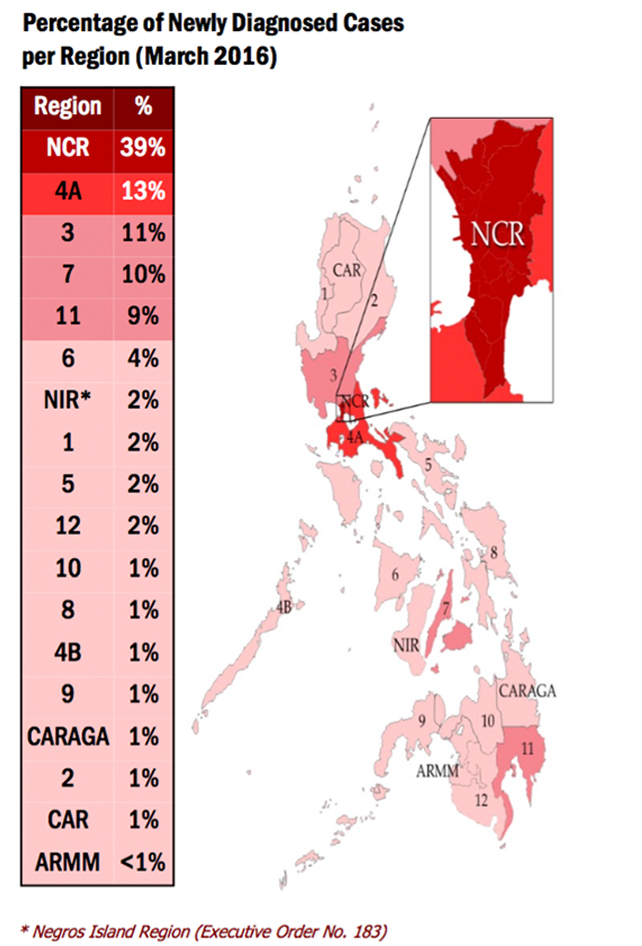
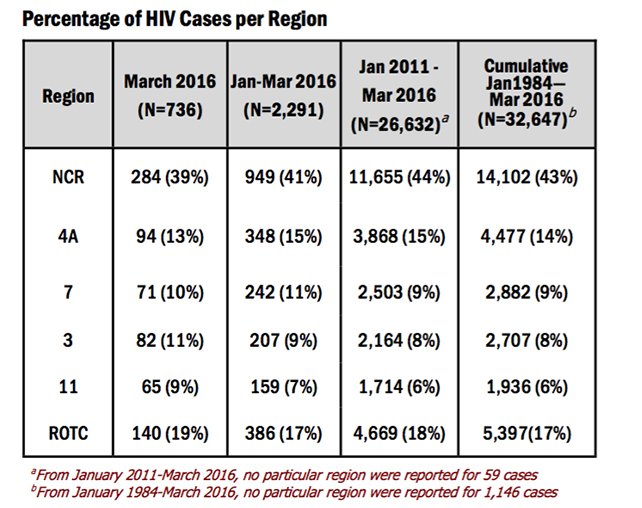
The regions with the highest number of reported cases for March were: National Capital Region (NCR) with 284 (39%) cases, Region 4A with 94 (13%) cases, Region 3 with 82 (11%) cases, Region 7 with 71 (10%) cases, and Region 11 with 65 (9%) cases.
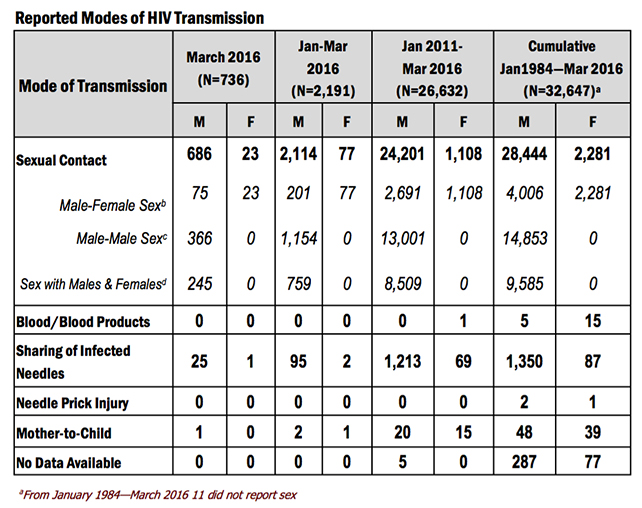
Reported modes of transmission were sexual contact (709), needle sharing among injecting drug users (IDU) [26], and mother-to- child transmission (1). Eighty-six percent (86%) of those transmitted through sexual contact were among males who have sex with males (MSMs).
The effect of HIV among the youth continue to be even more defined, with the March data showing 204 (28%) cases involving youth aged 15-24 years. Most (96%) were male. Ninety-nine percent (203) were infected through sexual contact (17 male-female sex, 110 male-male sex, 76 sex with both males and females), and 1 (<1%) through needle sharing among IDU.
Also becoming even more defined is the emergence of those who engage in transactional sex as a key affected population. People who engage in transactional sex are those who report that they pay for sex, regularly accept payment for sex or do both. In March, 12% (90) of the reported cases engaged in transactional sex. Most (96%) were male (Table 4) whose ages ranged from 18 years-54 years (median: 28 years) while 4 were female whose ages ranged from 23 years-34 years (median: 25 years). Forty percent (35) of males who engaged in transactional sex were the ones who paid for sex while 2 of the females engaged in both.
Also in March, there were 520 people living with HIV (PLHIV) who started taking antiretroviral medicine (as part of antiretroviral therapy). This was 16% higher than the same period last year (n=448). The median CD4 of these patients upon enrollment was 151 cells/mm3.
A total of 13,387 PLHIVs were presently on ART as of March 2016. Most (96%) were males. The median age of patients was 31 years (range: 11 months-77 years). Ninety-six percent were on first line regimen, 3% were on second line regimen and <1% were on mixed first and second line regimen.
For the month of March 2016, there were 26 reported deaths. Of the number, 92% (24) were male while 8% (two) were female. Fifteen (58%) of the reported deaths belong to the 25-34 year age group, eight were in the 35-49 year age group, two were youth aged 15-24 years old and one belongs to the 50 years and older age group. All were infected through sexual contact (four male-female sex, 14 male-male sex, eight sex with both males and females).